Exclusive: The truth about N95 surgical masks no one will mention...
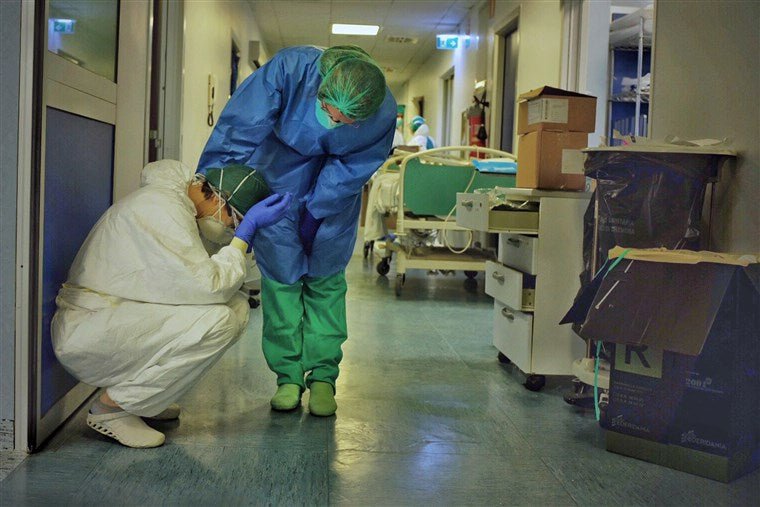

As the coronavirus spreads rapidly across the nation, most of us are aware there’s a shortage of personal protection equipment (PPE), primarily face masks, for our health care professionals and first responders.
Four weeks ago, few Americans probably knew what “N95” referred to, but now we do. It’s the industry standard model face mask used throughout the medical world, and now in dangerously short supply.
Of course, that’s not the whole story, and the facts are even more disturbing.
The primary manufacturer of the N95 mask, widely know for manufacturing ear protection, has outsourced all of its manufacturing to China. The special “melt-blown” fabric used to make the masks has skyrocketed in price. It was originally chosen because it was low cost, at $6,000 per ton, but because of high demand, is now selling for ten times that.
However, the most disturbing fact about the N95 mask is not that it’s in short supply, or that the U.S. must depend on China to purchase it.
The most disturbing fact is the efficacy of the N95 material has not been tested against the coronavirus specifically. Read that again: there has been no testing of how well N95 masks protect against the virus.
N95 respirator masks are able to filter out most airborne particles down to 0.3 microns in diameter. When worn correctly, the N95 blocks out at least 95 percent of small particles – hence the name.
However, the coronavirus is smaller.
According to researchers, the smallest coronavirus particles measure between 0.06 and 0.14 microns, meaning the N95 mask still isn’t proper protection against the virus.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30211-7/fulltext
No validation has been published on the effectiveness of N95 masks in a hospital environment. And yet huge corporations are in bed with the Chinese government to produce more masks and make more billions.
And for our medical professionals, it’s like using condoms manufactured with tiny holes.
There is an alternative.
In partnership with Bella Canvas, Nine Line has designed a low-cost disposable containment mask which can be rapidly produced using existing equipment. We are currently working through FDA testing laboratories to evaluate the effectiveness of chemical solutions and additives impregnated in our multilayer material. Current testing demonstrates the potential of preventing the spread of the virus by 99.99 percent.
Nine Line is prepared to completely turn over our production and cut-and-sew capability to the manufacture of these masks at cost.
But there is no time to waste.
We need to be able to accelerate FDA testing for the effectiveness of our proprietary
nano-technology material.
And we need government funding to keep our 200 people employed so we can make this happen.
The country has never needed American ingenuity more than it does at this moment. Nine Line stands ready to serve.





I just saw you on CNN. You rock! Thank you many times over. I’m basically speechless, but it is people like you who move the world forward.
I just sent your company link to President Trump in an email to his staff at The White House. Hang on tight buddy – you are NOT alone. I am working with the FDA on an EUA clearance for a ventilator accessory. Contact FDA and inquire about filing an EUA (Emergency Use Authority) for humanitarian clearance for COVID-19 Pandemic. Here is the email: DICE <dice@fda.hhs.gov> dice@fda.hhs.gov.
Here is the phone number:
EUA and IIE Resources:
Additional questions about the EUA or to submit a request, email CDRH-NonDiagnosticEUA-Templates@fda.hhs.gov.
Please review these resources and reach out to the appropriate contact listed below for further assistance. I hope this information is helpful. DICE staff are available via the internet at DICE@fda.hhs.gov and by phone at 1-800-638-2041 and FAX at 301-847-8149.
Industry Team
Division of Industry and Consumer Education
Office of Communication and Education
Center for Devices and Radiological Health
U.S. Food and Drug Administration
Tel: 1-800-638-2041
Email: DICE@fda.hhs.gov
RN -shared on FB!
Thank you for what you are all doing! I am an er nurse and my husband is a state trooper. We have two little kids and we dont want to bring this home to them. We need people and companies like you protecting us. How do I buy these masks?… I know you dont want money but I want to support this company for helping save our lives!
I have visited your store in Charleston SC. I have purchased from you online. I am a veteran and I am a Nurse. You have a customer for life. Thank you!!
Leave a comment